
 An element (with its unique atomic number) may have atoms with different numbers of
neutrons,
thus different mass numbers.
An element (with its unique atomic number) may have atoms with different numbers of
neutrons,
thus different mass numbers.
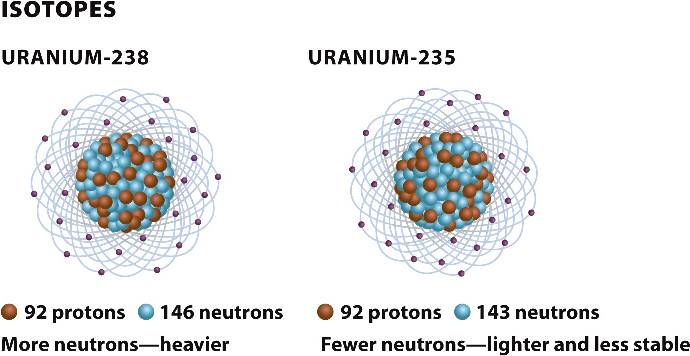
Atoms of an element with different mass numbers are called isotopes.
Uranium has an atomic number of 92; two isotopes are U-238 with 146 neutrons and U-235 with 143 neutrons.