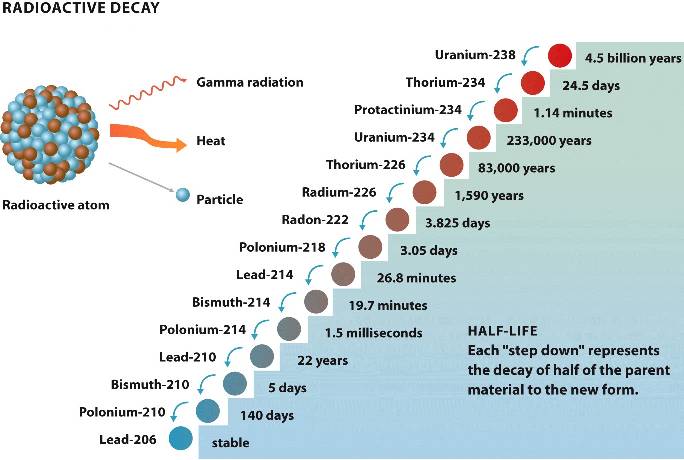
 Some isotopes are radioactive: the nucleus is unstable and spontaneously decays
into daughter atoms with release of subatomic particles
as well as harmful gamma
radiation
and heat.
Some isotopes are radioactive: the nucleus is unstable and spontaneously decays
into daughter atoms with release of subatomic particles
as well as harmful gamma
radiation
and heat.
This decay occurs at a constant rate expressed as half-life - the time it takes for half the parent material to decay to daughter material.
The radioactive decay of an isotope such as Uranium-238 may undergo many radioactive daughter isotopes before reaching a stable product such as Lead-206.