
 An element has unique chemical properties and is made of one kind of atom.
An element has unique chemical properties and is made of one kind of atom.
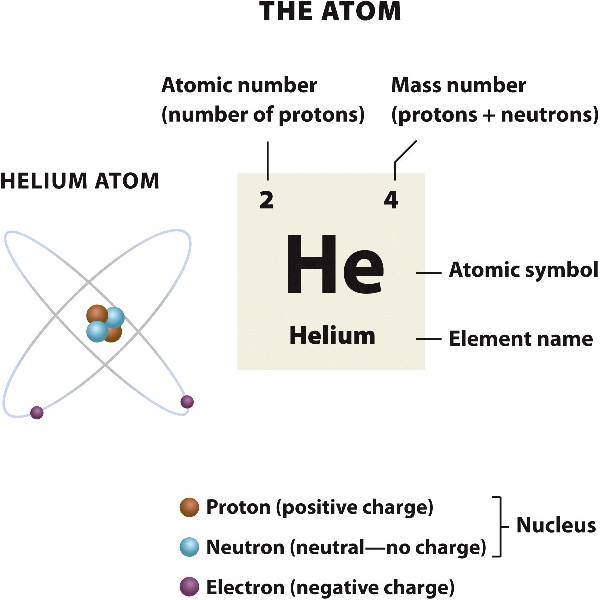
The nucleus of an atom contains positively charged particles called protons and particles with no charge called neutrons.
Electrons are negatively charged particles that orbit the nucleus.
Atoms are electrically neutral: they always have an equal number of protons and electrons.
The number of protons of an atom is its atomic number, and gives the element unique chemical properties.
The sum of the number of protons and neutrons make up its mass number.