
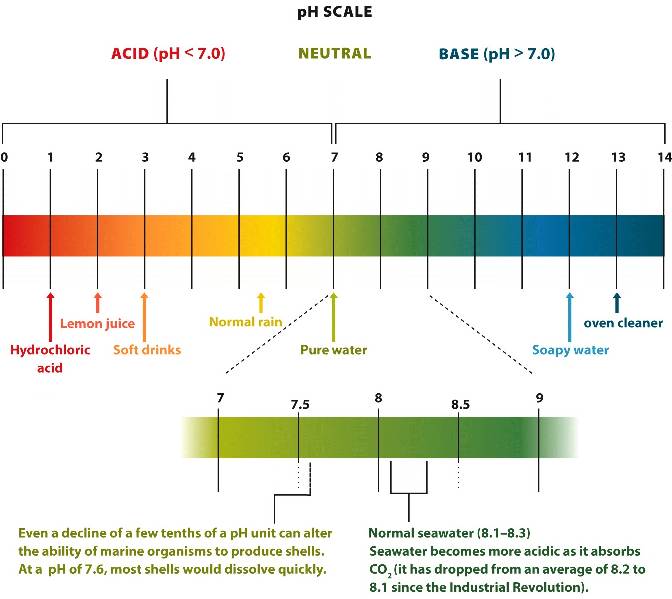
 Pure water
has the same concentration of hydrogen ions
and hydroxide ions
that is represented as 7
(neutral)
on a logarithmic pH scale.
Pure water
has the same concentration of hydrogen ions
and hydroxide ions
that is represented as 7
(neutral)
on a logarithmic pH scale.
A substance that increases concentrations of hydrogen ions has a pH lower than 7 and is called an acid.
A substance that lowers concentrations of hydrogen ions has a pH greater than 7 and is called a base.
An acidic solution has a high concentration of hydrogen ions with a low pH.
A basic (alkaline) solution has a low concentration of hydrogen ions with a high pH.