
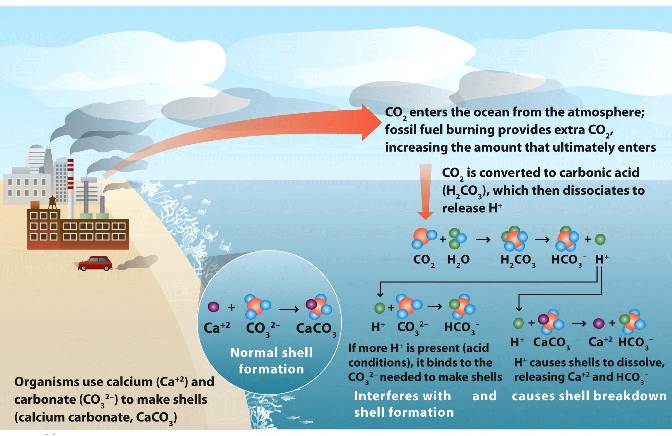
 CO2 dissolved in water forms carbonic acid (H2CO3).
CO2 dissolved in water forms carbonic acid (H2CO3).
Carbonic acid is in dynamic equilibrium with bicarbonate ion (HCO3-) and hydrogen ion (H+).
The excess H+ can bind to carbonate CO3--, the raw material for making calcium carbonate (CaCO3) shells of aquatic organisms.