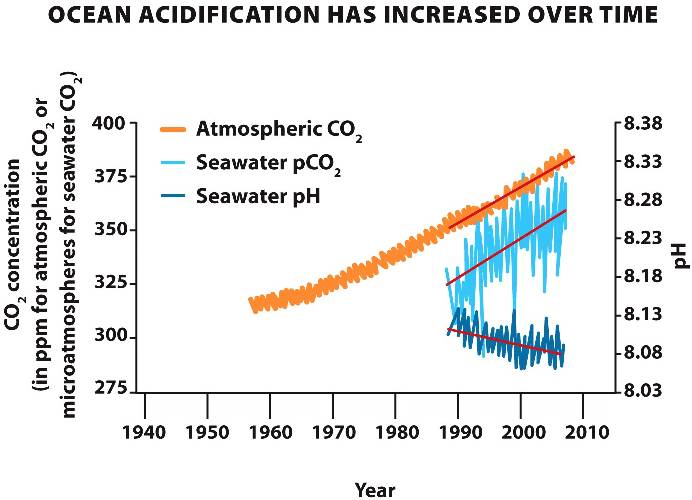
 The concentration of CO2 (carbon dioxide)
in the atmosphere has increased dramatically in the last 50 years.
The concentration of CO2 (carbon dioxide)
in the atmosphere has increased dramatically in the last 50 years.
When the excess CO2 dissolves in water, the pH of aquatic ecosystems decreases, leading to acidification of oceans and lakes.
Note that on the logarithmic pH scale, a decrease of 0.1 in pH is a 30% increase in hydrogen ion concentration.